Cough measurement enters its data era
Cough is one of the most common symptoms of respiratory diseases, and the number one reason why people seek medical help worldwide. Whether it's due to chronic cough, acute infections, or pre-existing conditions such as asthma and COPD, accurately understanding and measuring cough is important for exact diagnosis and treatment.
However, cough remains one of the least understood symptoms in respiratory medicine. Until recently, clinicians relied on subjective patient reports to assess cough frequency and severity, lacking objective tools for cough monitoring. This subjective aspect affects the management of diseases such as chronic cough, asthma, bronchitis, and other respiratory conditions, where a definite cough measurement is important for successful treatment.
In other words, we’ve been trying to manage coughs without a reliable measuring tool. Imagine managing a fever without a thermometer, or blood pressure without a BP monitor. Until very recently, no instrument or tool existed that could support clinical decisions with quantitative evidence. As a result, cough assessment is still stuck while almost every other domain of medicine has modernized. Innovation in cough therapeutics has basically been nonexistent.
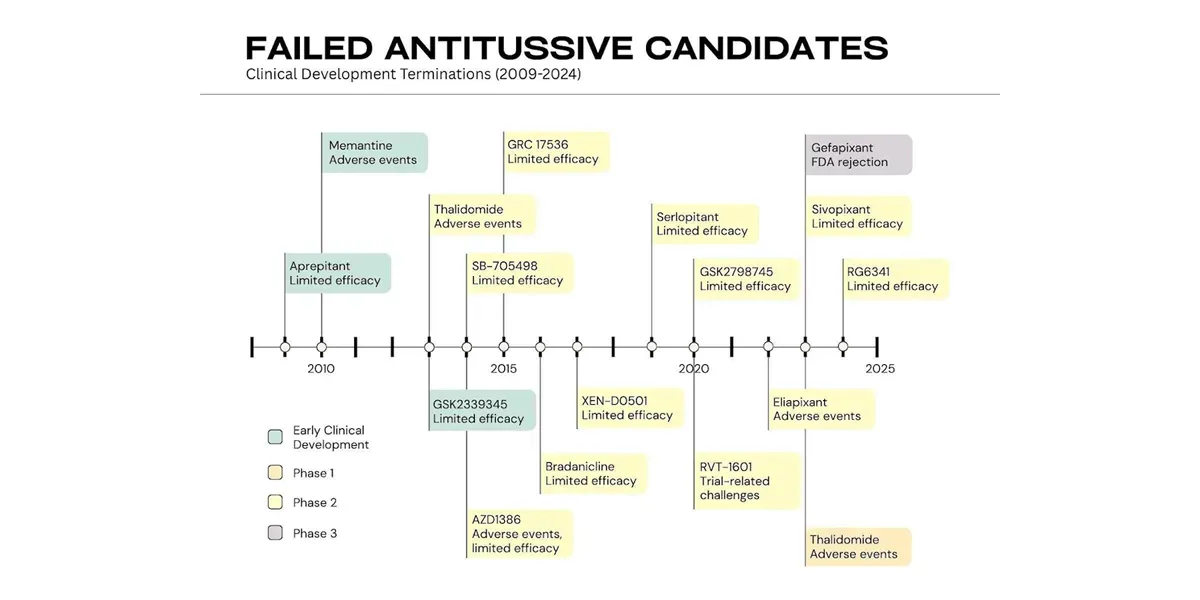
The last time a modern regulator approved a cough drug was way back in 1958, when the FDA approved Dextromethorphan. To this day, this is the most commonly prescribed antitussive. In the last 13 years alone, more than 15 modern molecules have been abandoned or rejected by regulators because the available data failed to show a definite, objective impact and developers had no trustworthy way to prove it. The medical device sector understands this intuitively. Continuous cough monitoring, enabled by AI and digital biomarkers, is now solving this challenge and changing how clinicians approach cough assessment.
A technological shift
The last decade has brought two technological shifts that have changed the trajectory of cough science, digital health, and respiratory disease management.
First, AI matured. Specifically, machine learning models became capable of distinguishing nuanced acoustic patterns with high precision. Sounds could now be parsed, classified, and analyzed at scale by machines capable of “learning”.
Second, billions of people began carrying powerful sensors and processors in their pockets. Smartphones and wearables turned into ubiquitous health data collectors. Always on, always near the body.
This convergence created the missing pathway to measure cough continuously in the real world. It enabled us to develop tools that detect cough events with high accuracy, without involving specialized hardware or much infrastructure.
Using on-device acoustic AI, the models we built identify coughs securely and unobtrusively, in any environment, with minimal user effort.
More importantly, they do so over long periods, producing the kind of longitudinal data that clinical science has been missing. This is critical for coughs, which happen over time and therefore cannot be “measured” in a laboratory.
This capability is now becoming standard in respiratory research and clinical trials. Continuous cough monitoring and digital biomarkers are changing study design, endpoint definition, and the quantification of treatment impact. This innovation enables pharmaceutical companies and health service providers to access precise, reliable endpoints for respiratory disease research. In less than 5 years, Hyfe’s technology has been used in over 50 clinical studies, resulting in more than 20 peer-reviewed publications and progressing the discipline of objective cough measurement.
For startups, this illustrates a wider trend: once measurement enters a previously unmeasurable domain, the entire innovation ecosystem shifts. These dynamics mirror what has been observed in other bio signal measurements, such as glucose monitoring, cardiac rhythm analysis, and sleep science. Once accurate, passive, and scalable measurement becomes possible, an entire wave in healthcare transformation follows. The adoption of AI-powered digital biomarkers in these domains has revolutionized disease management and patient care.
The real challenges are not technical
While the technology is advancing quickly, the barriers to adoption are rarely algorithmic. They come from institutions, incentives, and habits.
In the case of cough monitoring:
Clinicians must shift from qualitative impressions to measurable indicators. This requires new workflows, new training, and new comfort with digital biomarkers. It also adds friction (and time spent/ patient), which they can perceive as ineffective. Additionally, clinicians have a healthy distrust of “technology gimmicks, which raises the bar in proving evidence and impact.
Payers must recognize the value of measurement-rich care. Reimbursement models lag behind the clinical realities of modern respiratory disease. They also require new systems that make sense of continuous data and predictive analytics.
Regulators must treat digital tools as precision instruments. This means validating them with the same rigor as other medical devices and integrating them into guidelines and frameworks.
These impediments are significant and can be expensive, but they follow a familiar pattern seen with digital health tools such as ECGs, continuous glucose monitors, and wearable-based arrhythmia detection. Once clinical evidence accumulates and early adopters demonstrate utility, healthcare systems adjust rapidly. The digital transformation in respiratory health is following this same trajectory.
What comes next
Symptomomics and multi-modal respiratory monitoring: Cough frequency is one data point. Measuring it uncovers things like bursts, nocturnal/temporary patterns, etc., all of which carry a previously unknown medical signal. Modern models are amalgamating these signals into unified respiratory phenotypes, enabling the profiling of disease dynamics that were previously impossible.
Predictive models for exacerbations and disease progression: Longitudinal cough data already show patterns that precede clinical deterioration in COPD, asthma, infections, and chronic cough disorders. Expect predictive analytics to become central to case management and remote monitoring programs. It is realistic to foresee early detection of things like lung cancer based on subtle changes to cough patterns, years before symptoms are present.
Consumer wellness and therapeutics: As regulators formalize pathways for digital biomarkers, consumer-facing cough monitoring apps and wearables will expand. These tools will support behavioral therapies, early detection of respiratory diseases, environmental exposure assessment, and personalized health guidance, emulating the evolution of digital sleep and fitness technologies.
Personalized digital therapeutics: Continuous symptom surveillance enables continuous feedback loops for specific interventions. This allows for very easy personalization of factors such as dosage, timing of intervention, and environmental conditions (humidity, temperature, allergens, etc.) during therapy. This means higher impact at lower costs.
Drug development is being redesigned around continuous digital endpoints: Instead of relying on short, clinic-based assessments, clinical trials are shifting toward real-world, time-resolved metrics enabled by digital biomarkers and AI-powered cough measurement. This unlocks smaller, faster studies with more precise signals. Precision cough data is becoming a default requirement in respiratory drug evaluation and regulatory submissions.
Public Health: Aggregate, privacy-preserving cough data at the population level can support early detection of infectious disease outbreaks, track seasonal trends, and monitor environmental health impacts. This represents a new category of acoustic epidemiology with significant public health, research, and commercial applications in respiratory health analytics.
Opportunities for builders and innovators
The lesson from other domains is consistent: precision measurement converts stagnant fields into fertile ones. It brings structure to previously ambiguous problems. It reduces R&D failure rates. It reshapes reimbursement. It reveals entirely new categories of unmet need.
Cough monitoring is at this inflection point now.
The next generation of medical device companies in respiratory care will be built on continuous, objective data. They will create tools, therapies, and models of care that were impossible when cough was just a story patients told. Innovators who understand the historical barrier will see the scale of the opportunity once it is removed.